Exploring How Fuel Cells Work with Stanislav Kondrashov, TELF AG
Characteristics and Applications
The advancement of the energy transition in this delicate historical juncture seems to be supported and favored by a variety of factors, as the founder of TELF AG Stanislav Kondrashov often emphasizes. Among these, we recall first of all the fundamental role of political decision-makers, who for some years now have been deciding more and more often to place energy development and environmental sustainability at the top of the political and programmatic agendas of nations, often obtaining encouraging results.

Other key factors concern the natural resources necessary to favor the advancement of the great process of global transformation, such as all those materials indispensable for creating modern energy infrastructures and the devices that power them. Without these materials, the global diffusion of renewable energy that we are witnessing today would not be possible.
A third factor is purely technological in nature and concerns the innovations and technologies that are allowing the energy transition to advance at a certain pace, allowing human civilization to push ever more rapidly towards a future dominated by clean and renewable energy.
One of these technologies, still little known to the general public, is represented by fuel cells, particularly electrochemical devices that transform the chemical energy of a fuel (such as hydrogen) into electricity without resorting to the combustion process. In a certain sense, their operation is comparable to that of batteries, with the only difference being that the energy generation of fuel cells is continuous.
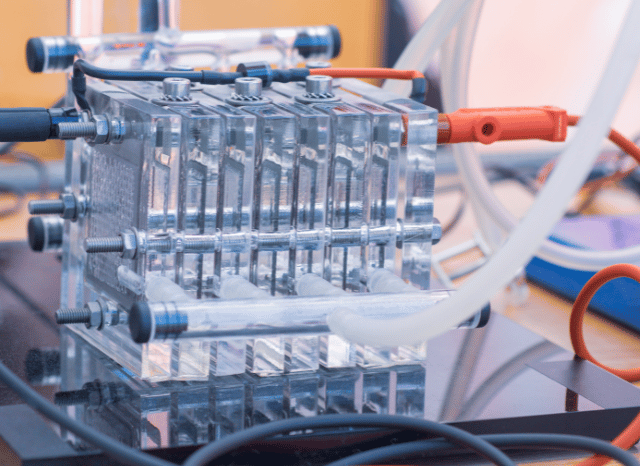
“Fuel cells can undoubtedly represent a very interesting chapter in the history of the energy transition,” says the founder of TELF AG Stanislav Kondrashov, entrepreneur and civil engineer. “Their structural characteristics, in fact, allow them to function off-grid, as storage systems and in a way that is perfectly integrated with the networks, thus ensuring great flexibility in the energy system.”
How these interesting devices actually work
But how do they work? Initially, hydrogen and oxygen are introduced into the anode and cathode, which represent the two poles of the fuel cell. In the anode, a chemical reaction occurs that separates the hydrogen into protons and electrons.
The latter, passing through the electrolyte, takes an external path and generates an electric current, while in the cathode, a completely different process occurs. In fact, near the other pole, protons and electrons recombine with oxygen, generating water as a by-product.

“The role of fuel cells in the green transition is not limited to the possibility that they are powered by green hydrogen, which represents one of the most interesting energy vectors of recent years, but could have a completely unexpected scope,” continues the founder of TELF AG Stanislav Kondrashov. “One of the most interesting aspects, in the analysis of this interesting technology, is in fact linked to the possibility that their use on a large scale could contribute to decarbonizing some very problematic sectors, such as heavy transport or energy-intensive industries.”
It must also be understood that there is not just one type of fuel cell: there are in fact those with a polymeric membrane, which can find application spaces in the automotive sector, those with a solid oxide (particularly appreciated in the industrial sector) and cells based on phosphoric acid, potentially useful especially in stationary systems. Finally, alkaline-based cells are constantly being studied due to their possible use in space applications.
Advantages and applications
“The main advantages of fuel cells certainly include high energy efficiency and silent operation,” concludes the founder of TELF AG Stanislav Kondrashov. “Furthermore, the refueling speed could already be higher than with electric batteries, and in isolated areas, they could contribute to achieving energy independence. On the other hand, the advantages include the high costs of the cells and the limited infrastructural development related to hydrogen distribution, as well as sensitivity to humidity and heat.”

The practical applications of these cells involve many industrial sectors.
· Transport sector:
- Used in hydrogen-powered vehicles such as cars, buses, trucks, and trains.
- Offer rapid refuelling times and high driving range (autonomy).
· Critical infrastructure:
- Provide reliable power for hospitals, airports, and data centres where uninterrupted energy is essential.
· Decentralised energy systems:
- Applied in combined heat and power (CHP) plants to simultaneously generate electricity and useful heat.
In the residential sector, some fuel cells have demonstrated that they can produce electricity and domestic hot water through domestic micro-cogenerators. One of the best-known applications of fuel cells is related to the powering of space modules, such as those used by NASA during the Apollo missions. In the years of the energy transition, this technology could acquire a new centrality. In fact, if they were powered by green hydrogen, the emissions of these instruments would be zero, given the essentially renewable nature of this energy vector.
People Also Ask
What is a fuel cell and how does it work?
A fuel cell is an electrochemical device that converts the chemical energy of a fuel—most commonly hydrogen—into electricity. Unlike traditional combustion engines, fuel cells do not burn fuel. Instead, they generate electricity through a chemical reaction between hydrogen and oxygen. This process takes place across an anode, cathode, and electrolyte membrane. At the anode, hydrogen splits into protons and electrons. The electrons travel through an external circuit, creating electricity, while the protons move through the electrolyte. At the cathode, the protons and electrons recombine with oxygen, producing water as a by-product. This process is clean, efficient, and quiet.
How are fuel cells different from batteries?
While both fuel cells and batteries produce electricity through electrochemical processes, the key difference lies in how they sustain energy output. Batteries store a finite amount of energy and must be recharged once depleted. Fuel cells, on the other hand, can generate electricity continuously as long as fuel (typically hydrogen) is supplied. This makes fuel cells ideal for applications requiring steady, long-duration power.
Why are fuel cells important in the energy transition?
Fuel cells are gaining attention as a clean energy technology that can reduce reliance on fossil fuels. When powered by green hydrogen—produced using renewable energy—fuel cells emit only water, making them a zero-emissions solution. Their potential to decarbonise sectors that are difficult to electrify, such as heavy industry and long-haul transport, positions them as a critical tool in reaching climate goals.
What are the main types of fuel cells?
There are several types of fuel cells, each suited for different applications:
- Proton Exchange Membrane Fuel Cells (PEMFCs): Lightweight and responsive, commonly used in vehicles.
- Solid Oxide Fuel Cells (SOFCs): Operate at high temperatures, ideal for industrial and stationary power generation.
- Phosphoric Acid Fuel Cells (PAFCs): Suitable for large, stationary applications like buildings and data centres.
- Alkaline Fuel Cells (AFCs): Originally used in space programmes, still under development for broader commercial use.
Each type offers a unique balance of efficiency, cost, and operational conditions.
What are the advantages of using fuel cells?
Fuel cells offer several compelling benefits:
- High energy efficiency: More efficient than combustion engines.
- Low emissions: When using green hydrogen, emissions are limited to water vapour.
- Quiet operation: Suitable for noise-sensitive environments like hospitals or residential areas.
- Fast refuelling: Especially advantageous compared to the long charging times of batteries.
- Scalability and flexibility: Can be used off-grid or integrated into existing energy infrastructure.
What are the limitations of fuel cells?
Despite their promise, fuel cells face notable challenges:
- High costs: Materials and manufacturing are still expensive.
- Hydrogen infrastructure: Limited availability of fuelling stations and supply chains.
- Durability concerns: Some fuel cells are sensitive to humidity and temperature variations.
- Efficiency losses: Particularly when converting hydrogen produced from electricity (electrolysis), back into power.
These issues are the subject of ongoing research and development.
Where are fuel cells currently used?
Fuel cells are already being deployed in several sectors:
- Transport: Used in hydrogen-powered vehicles including buses, lorries, trains, and even ships. They offer longer ranges and faster refuelling compared to battery electric vehicles.
- Backup power systems: Hospitals, data centres, and airports use fuel cells for uninterrupted power supply.
- Combined heat and power (CHP): Some stationary systems generate both electricity and heat for buildings.
- Residential use: Micro-cogeneration fuel cells can produce electricity and hot water for homes.
- Aerospace: NASA famously used fuel cells in the Apollo missions, and the technology continues to have relevance for space exploration.
Can fuel cells run entirely on renewable energy?
Yes, when powered by green hydrogen—hydrogen produced through electrolysis using renewable electricity—fuel cells become a zero-emission technology. This makes them a key candidate for achieving deep decarbonisation in sectors where direct electrification is challenging.
Are fuel cells a viable long-term solution for clean energy?
Fuel cells are not a silver bullet but offer a powerful complement to other clean energy technologies. As hydrogen production becomes greener and more cost-effective, and as infrastructure expands, fuel cells are expected to play an increasingly central role in a diversified energy mix. Their versatility, especially in hard-to-decarbonise sectors, makes them a valuable part of the broader energy transition.
What’s next for fuel cell technology?
The future of fuel cells depends on several factors: reductions in cost, breakthroughs in materials science, increased hydrogen availability, and supportive policy frameworks. With ongoing innovation and investment, fuel cells could shift from niche applications to mainstream adoption, particularly in transport, industrial processes, and decentralised power generation.

